Renewable Energy: Salt Water Fuel Cell Science Kit









Renewable Energy: Salt Water Fuel Cell Science Kit
Renewable Energy: Salt-water Fuel Cell Science Kit
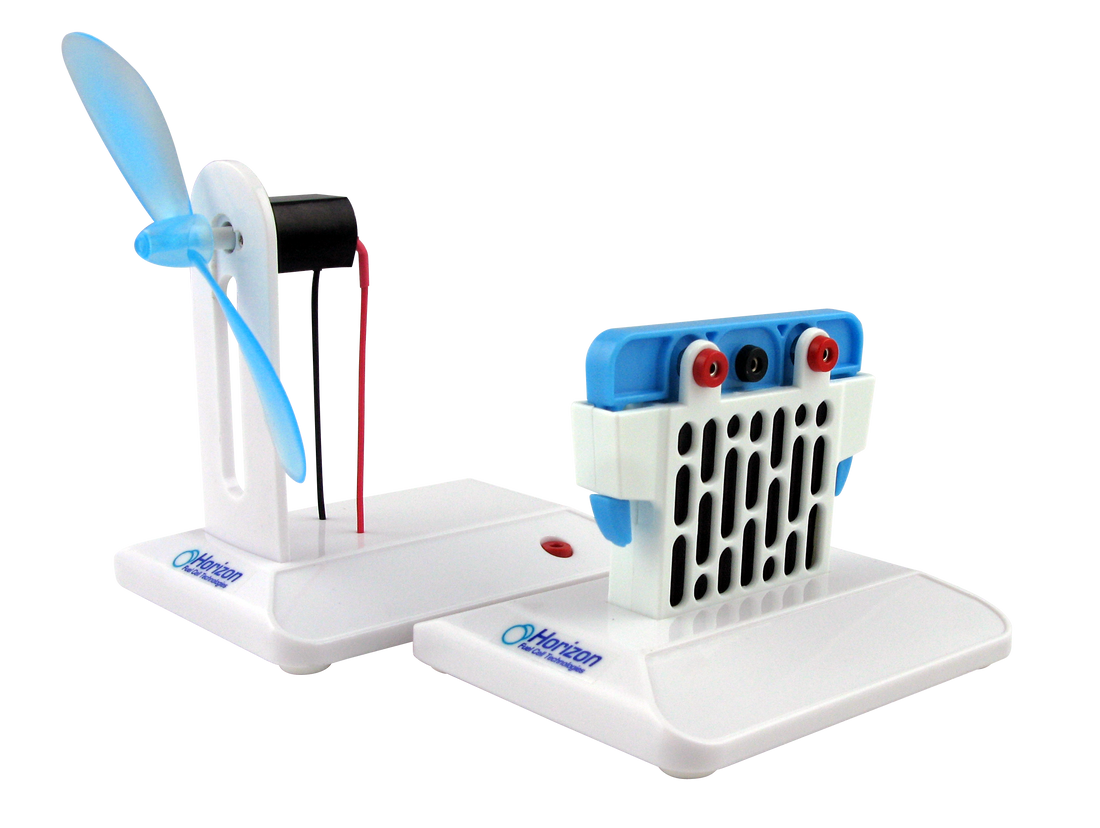
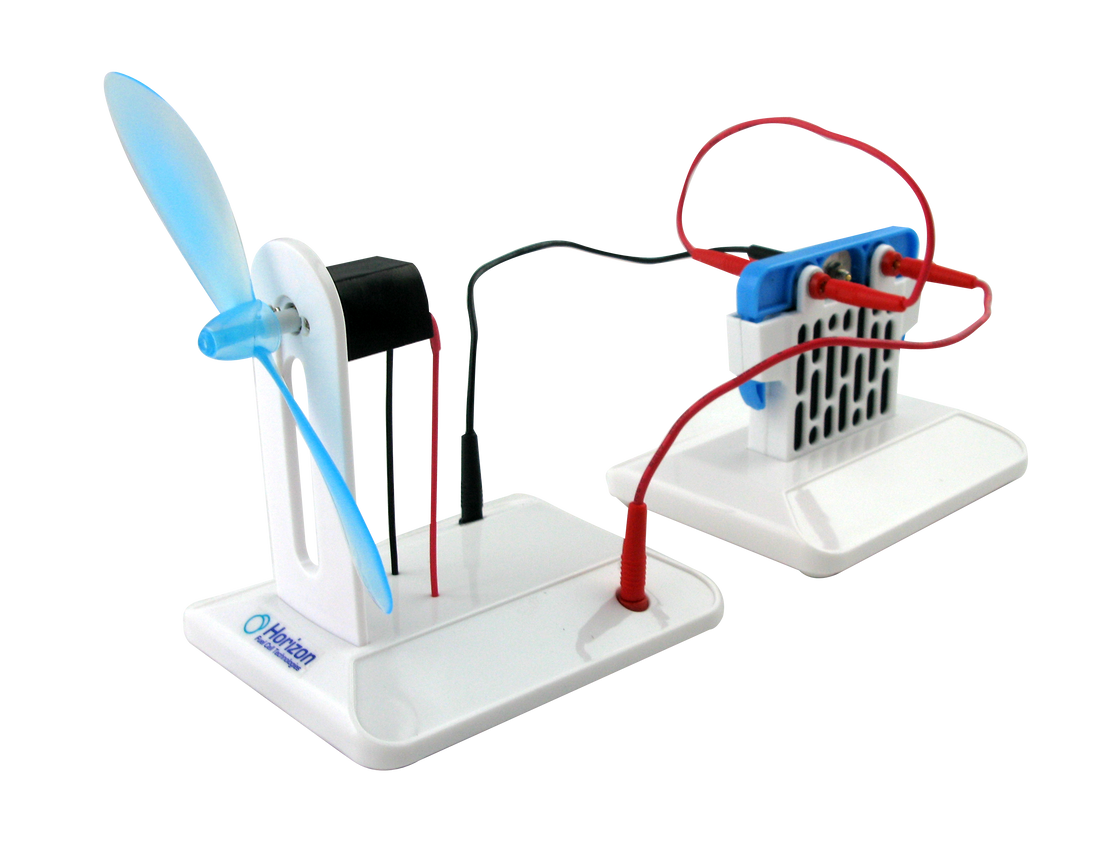
The Salt Water Fuel cell kit enables you to investigate the science behind salt water fuel cell technology, either by powering the included mini-turbine or creating your own power applications.
- Features magnesium salt-water fuel cell power device
- Develop your own micro-fuel cell applications
- Easy to integrate and use
- Compatible with other Horizon science kits
- Create energy from salt water solution and power a fan
- Analyze current and voltage variation using different salt concentrations
- Analyze current and voltage variations using different temperatures
- Analyze current and voltage variations using different fuel volumes
What is a salt water fuel cell? How does it work?
Salt Water Fuel Cell Kit: Exploring Sustainable Energy Solutions
The Salt Water Fuel Cell Kit provides an engaging way for students to investigate the science behind salt water fuel cell technology. This innovative kit allows for hands-on experimentation, enabling learners to power the included mini-turbine or create their own energy applications using salt water as a renewable energy source.
Key Features:
-
Magnesium Salt-Water Fuel Cell: The kit includes a magnesium-based power device that demonstrates how salt water can be transformed into electricity.
-
Micro-Fuel Cell Applications: Students can develop their own micro-fuel cell projects, encouraging creativity and innovation in renewable energy solutions.
-
Ease of Use: The kit is designed for easy integration into educational settings, making it accessible for students of various ages and backgrounds.
-
Compatibility: Fully compatible with other Horizon science kits, this kit can enhance a variety of STEM activities.
-
Power Generation: Students can create energy from a salt water solution to power a fan, illustrating the practical applications of fuel cell technology.
-
Experimental Analysis: Engage in hands-on experiments to analyze current and voltage variations based on:
- Different salt concentrations
- Various temperatures
- Different fuel volumes
If the cell is linked to an electrical circuit, the free electrons will pass inside it in order to reach the cathode and let the second reaction happen. You can find the two half redox equations below.
Mg + 2H2O → Mg(OH)2 + 2H + 2e-
1/2 O2 + H2O + 2e- → 2OH
2Mg + O2 + H2O → Mg(OH)2
The salt in the water is used as a catalyst. In other words, it means that salt accelerate the reaction between Mg and water.
Salt-water Fuel Cell Experiments
Experiment 1 : Create electricity from water salted solution
Experiment 2 : Using different salt concentrations
Experiment 3 : Using different water temperature
Experiment 4 : Using different fuel volume
Estimated Delivery:
- Processing time: 1-2 business days
-
Shipping time:
- Ireland: 15 - 20 business days
Please note: Delivery times may vary during peak seasons or due to unforeseen circumstances.